42 phase labels in chemical equations
3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g) Chapter 7 Write the formula and identify the phase for the product (s) and balance the following reaction. Na 2 SO 4 (aq) + CaCl 2 (aq) ---> Since this is a double replacement reaction we can write the formulas of the products by exchanging the cations and anions. When you do this remember to keep track of the individual charges on the cations and anions.
Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases.

Phase labels in chemical equations
Solved 1. Write balanced chemical equations using proper | Chegg.com Chemistry questions and answers. 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. b) Solid calcium carbonate is added to a solution of hydrochloric acid, yielding an aqueous solution ... Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet Phase Transitions: Melting, Boiling, and Subliming - Lardbucket.org Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O(s) → H 2 O(ℓ) No chemical change is taking place; however, a physical change is taking place.
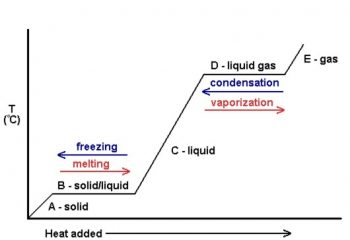
Phase labels in chemical equations. Enthalpy (Heat) of Reaction and Thermochemical Equations - JoVE A balanced chemical equation that includes phase labels and enthalpy of reaction, Δ H, is called a thermochemical equation. Consider the combustion of methane - a primary source of fuel. Burning methane releases heat to the surroundings. The exothermic nature of the reaction is indicated by the negative enthalpy change in the thermochemical ... 6.3 Acid-Base Reactions - CHEM 1114 - BCcampus The process represented by this equation confirms that hydrogen chloride is an acid. When dissolved in water, H 3 O + ions are produced by a chemical reaction in which H + ions are transferred from HCl molecules to H 2 O molecules ().. Figure 1. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and ... End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation. State symbols and phase changes - StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
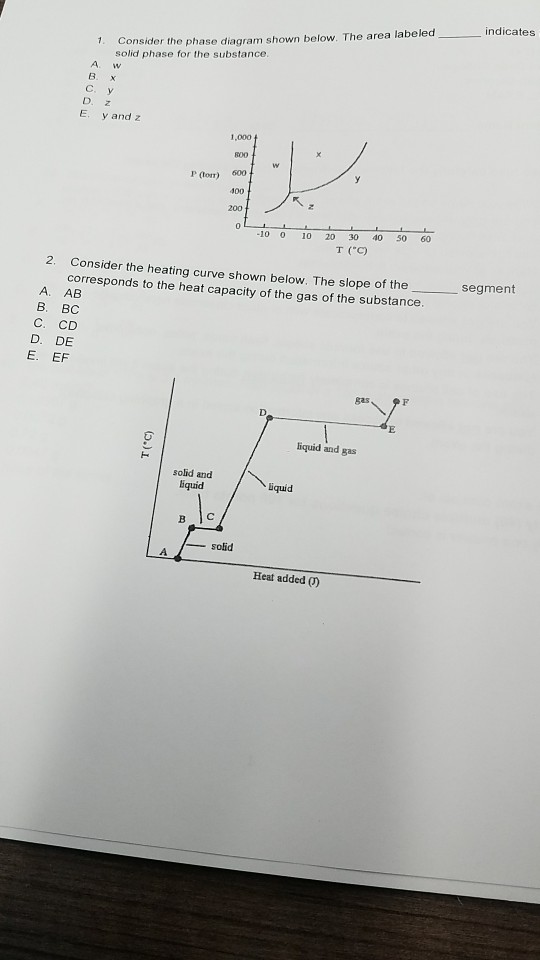
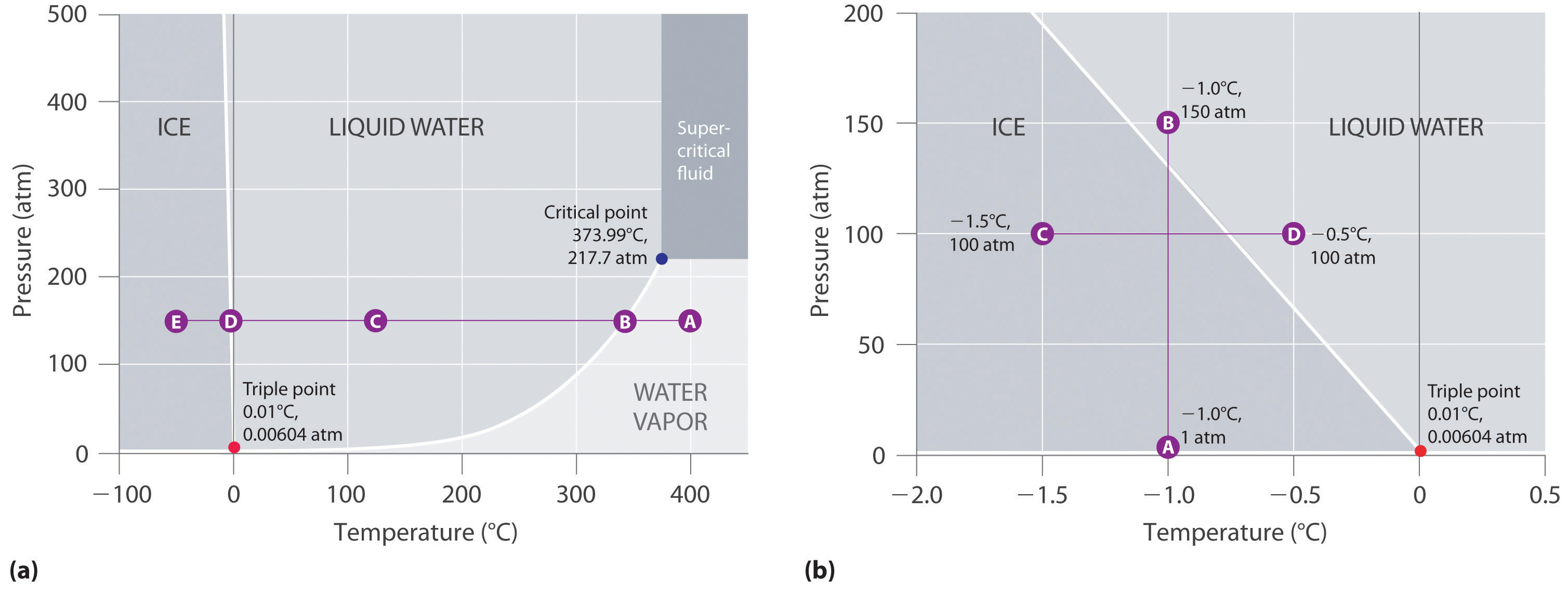
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution 5.1 The Chemical Equation - Introductory Chemistry - NSCC In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ... Phase Definition and Examples - ThoughtCo A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such ... chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution
Chapter 4 Section E Neutralization Reactions - PEOI Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl(aq) and Fe(OH) 3 (s) still proceeds according to the equation 3HCl(aq) +Fe(OH) 3 (s) --> 3H 2 O(l) +FeCl 3 (aq) even though Fe(OH) 3 is not soluble. When one realizes that Fe(OH) 3 (s) is a component of rust, this explains why ... Conventions for Writing Chemical Equations - Arrows, Phases ... - YouTube presents: Conventions for Writing Chemical Equations including Arrows, Phases, Coefficients, Reactants, Products and moreTired... End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
A thermochemical equation is a balanced chemical reaction equation ... An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. The following four reactions are examples of thermochemical equations. The first two are exothermic and the last two are endothermic reactions. 2 H 2 (g) + O 2 (g) ----> 2 H 2 O(l) DH = -571.6 kJ
DOC Chapter 2 Identify the reactants and products in a chemical equation. Write chemical equations using appropriate phase labels, symbols of reaction conditions, and the presence of a catalyst. 2.10 Balancing Chemical Equations. Learning Objectives. Determine if a chemical reaction is balanced. Master techniques for balancing chemical equations. (Example 2.12)
PDF Chemical reaction : conversion of substances into different substances ... Phase labels : show the phase of reactants or products (s) : (l) : (g) : (aq ) : Chapter 7: Chemical Reactions ch7a Page 1 ___ H 2+ ___ O 2 → ___ H 2O Law of conservation of mass: 1. Leave elemental substances for last: ... Use the chemical equations worksheet to practice writing and balancing chemical equations.
End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
PDF aq - Anoka-Ramsey Community College • Predict possible products with phase labels • Write the balanced chemical equation. If no visible reaction occurs, write NR. If a precipitate forms, give the formula and name of the precipitate. • Write the complete ionic equation and net ionic equation for all reactions (whether or not a visible reaction occurs) 1.
Phase Diagram | Explanation, Definition, Summary & Facts The first curve line (A, T) separated the solid and vapour phase, second curve line (T, B) separates the solid and liquid phase whereas third curve (T, C) separates the liquid and vapour phases of a substance (Fig. 1).
The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).

😀 Zn cuso4 net ionic equation. Help writing complete ionic and net ionic equations.? Hg2(NO3)2 ...
Phase Transitions: Melting, Boiling, and Subliming - Lardbucket.org Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O(s) → H 2 O(ℓ) No chemical change is taking place; however, a physical change is taking place.
Solved Part 7: Aqueous Chemistry...Ionic Equations 7. In | Chegg.com In many aqueous chemical reactions, there are ions that are not involved in the chemical change but serve to deliver the ions that are involved. hen a compound in a chemical equation has an aqueous label, and is ionic, it consists of the constituent ions separated in solution. We represent the ions in a complete This question hasn't been solved yet
Solved 1. Write balanced chemical equations using proper | Chegg.com Chemistry questions and answers. 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. b) Solid calcium carbonate is added to a solution of hydrochloric acid, yielding an aqueous solution ...


![Properties of Liquids – Introductory Chemistry, 1st Canadian Edition [Clone]](https://opentextbc.ca/introductorychemistryclone/wp-content/uploads/sites/291/2019/08/example-question-phase-diagram-1.png)







Post a Comment for "42 phase labels in chemical equations"